Abstract

Introduction: Chimeric antigen receptor T-cell therapy (CAR-T) directed against B-cell maturation antigen (BCMA) is a highly effective treatment for patients with relapsed/refractory multiple myeloma (RRMM). Prolonged cytopenias following CAR-T for lymphoid malignancies are common (PMID 33928266) and often preclude patients who progress after CAR-T from participating in clinical trials. However, there is limited understanding of the prevalence, severity and underlying mechanism of cytopenias following BCMA-directed CAR-T in RRMM. We here study the pattern of hematologic recovery and associated factors in this population.
Methods: We retrospectively reviewed laboratory data for 90 RRMM patients from our institution who received a BCMA-directed CAR-T product as part of a clinical trial between 2017 & 2021. Examining serial peripheral blood counts for these patients, we calculated the point prevalence of cytopenias at baseline (day 0) and at various time intervals following CAR-T infusion (30, 60, 90, 120, 150, 180, 270, 360, 540 & 720 days). Laboratory data was censored at the time of disease progression, and cytopenias were graded according to the Common Terminology Criteria for Adverse Events. We then sought to identify traits associated with increased risk of developing prolonged, high-grade, post-CART cytopenias. Since the median time to recovery from grade ≥3 cytopenias after lymphodepleting therapy in cilta-cel (PMID 34175021) & ide-cel (PMID 33626253) trials was approximately 2 months (range ~1-4 months), we compared baseline clinical and bone marrow characteristics of patients with one or more persistent grade ≥3 cytopenia four months after CAR-T infusion ("poor" hematologic recovery group) against those of patients with normal or less-severe cytopenias at that time point "(adequate" hematologic recovery group). Chi-Square, Fisher's Exact test and logistic regression models were used for statistical comparisons.
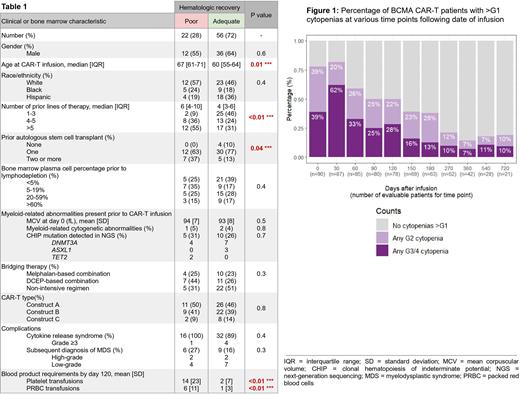
Results: The prevalence of any ≥G3 cytopenia (anemia, thrombocytopenia and/or neutropenia) at day 0, 60, 120, 180 & 360 post-CART was as follows: 39%, 33%, 28%, 13% & 7% (Figure 1). At the same time points, the prevalence of bicytopenia or pancytopenia (i.e., two or more lineages affected by ≥G3 cytopenias) was 14%, 19%, 10%, 6% & 2%, respectively. 78 patients (87%) were evaluable at the four-month cutoff; hematologic recovery, as defined above, was considered to be "poor" in 22 (28%) and "adequate" in the remaining 56 (72%). A comparison of baseline clinical & bone marrow characteristics for these two groups is shown in Table 1. Patients in the "poor" hematologic recovery group were older (median age at CAR-T infusion 67 vs 60 years, p=0.01), more heavily pre-treated (median number of prior lines of therapy 6 vs 4, p=0.004), and more likely to have received ≥1 prior autologous stem cell transplant (ASCT) (37 vs 13%, p=0.04). In a multivariate logistic regression model, having >3 prior lines of therapy had the strongest association with "poor" hematologic recovery at four months (multivariate odds ratio [OR] 8.3, 95% CI 1.5-76, p=0.03). Type of CAR-T construct and type of bridging chemotherapy were not associated with significant differences in time to hematologic recovery. Bone marrow findings at baseline were grossly comparable between the two groups, including plasma cell percentage & myeloid-related cytogenetic/genomic abnormalities. A considerably higher proportion of patients in the "poor" hematologic recovery group was subsequently diagnosed with myelodysplastic syndrome (27 vs 16%), but the difference did not reach statistical significance.
Conclusion: To our knowledge, this is the largest and most comprehensive characterization of prolonged, unexplained cytopenias in RRMM patients treated with BCMA-directed CAR-T. Roughly one third of patients had an ongoing ≥G3 cytopenia four months after infusion, but the majority recovered at one year (prevalence <10%). We found older age, higher number of prior lines of therapy, and prior history of ≥1 ASCT to be significantly correlated with poor hematologic recovery at four months post-CART, suggesting that T-cell redirection in RRMM patients with reduced bone marrow reserve due to aging and/or treatment-related toxicity may contribute to the decline of hematopoietic function by an unknown mechanism. Further research is needed to elucidate the biological basis for post-CART cytopenias in RRMM patients.
Disclosures
Lancman:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Richard:Janssen: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; C4 Therapeutics: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Rossi:BMS: Consultancy; janssen: Consultancy; adaptive: Consultancy; sanofi: Consultancy; gsk: Consultancy. Chari:Celgene, Novartis, Amgen, Janssen Oncology, Seattle Genetics, Bristol-Myers Squibb, Karyopharm Therapeutics, Genzyme, Oncopeptides, Takeda, Antengene, GlaxoSmithKline and Secura Bio: Consultancy; Celgene, Novartis, Janssen, Pharmacyclics, Amgen, Seattle Genetics and Takeda: Research Funding. Rodriguez:Janssen, BMS, Takeda, AbbVie, karyopharm, Artiva: Consultancy, Speakers Bureau. Cho:Takeda: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding; BMS/Celgene: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding. Richter:Oncopeptides: Consultancy, Honoraria; Secura Bio: Consultancy, Honoraria; Takeda: Consultancy; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hoffman:Abbvie: Other: Chair DSMB, Research Funding; Turning Point: Research Funding; Novartis: Other: Chair DSMB; Silence Therapeutics: Consultancy; Protagonist Therapeutics: Consultancy; Ionis: Consultancy; Repare: Research Funding; Scholar Rock: Research Funding; Novartis: Research Funding. Jagannath: Sanofi: Consultancy; Karyopharm: Consultancy; Janssen Pharmaceuticals: Consultancy; Legend Biotech: Consultancy; Takeda: Consultancy; BMS: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.